Ethyl-for-methyl substitution enhances the subtype specificity of mecamylamine analogues†
Abstract
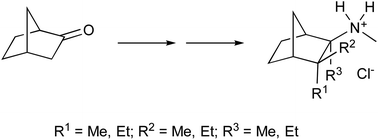
The synthesis of novel mecamylamine analogues is described in which one, two or three of the methyl groups of mecamylamine have been systematically replaced with ethyl groups. Assessment of the compounds highlights that simple ethyl for methyl changes changes to the parent structure can dramatically enhance activity and selectivity towards either the α4β2 (at the expense of α3β4) or the α3β4 (at the expense of α4β2) nicotinic acetylcholine receptor sub-type as compared to the parent compound.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...