Optimization of interstrand interactions enables burn detection with a collagen-mimetic peptide†
Abstract
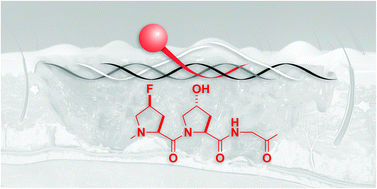
Collagen is an abundant component of the extracellular matrix and connective tissues. Some collagen-mimetic peptides (CMPs) that do not form homotrimers can anneal to damaged tissue. Here, through a computational screen, we identify (flpHypGly)7 as an optimal monomeric CMP for heterotrimer formation. We find that (flpHypGly)7 forms stable triple helices with (ProProGly)7 but not with itself. The nonnatural amino acid HflpOH, which is (2S,4S)-4-fluoroproline, is not toxic to human fibroblasts or keratinocytes. Conjugation of (flpHypGly)7 to a fluorescent dye enables the facile detection of burned collagenous tissue with high specificity. The ubiquity of collagen and the prevalence of injuries and diseases that disrupt endogenous collagen suggests widespread utility for this approach.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...