A gold-triggered dearomative spirocarbocyclization/Diels–Alder reaction cascade towards diverse bridged N-heterocycles†
Abstract
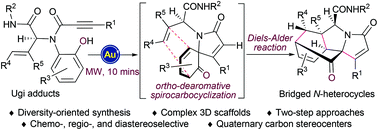
A rapid approach for the diversity-oriented synthesis of complex bridged polycyclic N-heterocycles from readily available starting materials in two operational steps has been developed. This strategy firstly introduces molecular diversity by an Ugi four-component reaction, and then achieves these bridged N-heterocycles via an efficient gold-triggered chemo- and diastereoselective cascade non-oxidative ortho-dearomative spirocarbocyclization/Diels–Alder reaction sequence. The application of microwave irradiation for this cascade process efficiently shortens the reaction time to 10 minutes and improves the diastereoselectivity.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...