Solvent interception, heterocyclization and desilylation upon NBS-induced sulfamidation of trimethyl(vinyl)silane†
Abstract
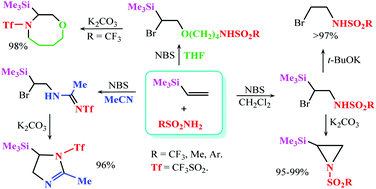
The reaction of trimethyl(vinyl)silane with sulfonamides in the presence of N-bromosuccinimide was shown to proceed regioselectively in methylene chloride under mild conditions and led to the products of bromosulfamidation in up to 88% yield. The obtained adducts undergo base-promoted dehydrobromination to give 2-trimethylsilyl-N-sulfonyl aziridines in a close to quantitative yield. In the reaction with trifluoromethanesulfonamide in acetonitrile or tetrahydrofuran, the Ritter-type (solvent-interception) products were obtained and converted to 1-triflyl-2-methyl-5-(trimethylsilyl)-2-imidazoline or 4-triflyl-3-(trimethylsilyl)-1,4-oxazocane in almost quantitative yield.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...