Regioselective radical arylation: silver-mediated synthesis of 3-phosphorylated coumarins, quinolin-2(1H)-one and benzophosphole oxides†
Abstract
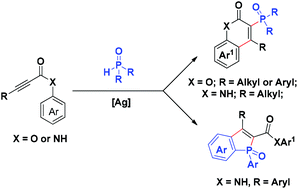
A silver-mediated domino radical addition/cyclization reaction of diarylphosphine oxides with propynoic acid derivatives is described in this article. The reaction of diarylphosphine oxides with alkynoates or propynoic amide was regioselective, producing 3-phosphorylated coumarins and benzophosphole oxides in moderate to good isolated yield, respectively. The hydrogen bond between P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–H was proposed to assist the vinyl radical regioselective arylation process. The reaction conditions were also compatible with the conversion of dialkylphosphine oxides to 3-phosphorylated-coumarins and quinolin-2(1H)-one in good yields.
O and N–H was proposed to assist the vinyl radical regioselective arylation process. The reaction conditions were also compatible with the conversion of dialkylphosphine oxides to 3-phosphorylated-coumarins and quinolin-2(1H)-one in good yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...