Rapid and efficient synthesis of a novel cholinergic muscarinic M1 receptor positive allosteric modulator using flash chemistry†
Abstract
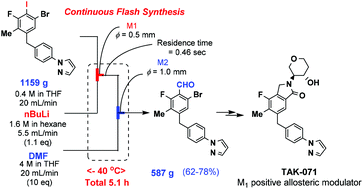
Continuous flow-flash synthesis of a 2-bromobenzaldehyde derivative 18 as a key intermediate of a novel cholinergic muscarinic M1 positive allosteric modulator 1 bearing an isoindolin-1-one ring system as a pharmacophore has been achieved using flow microreactors through selective I/Li exchange of 1-bromo-2-iodobenzene derivative 17 with BuLi and subsequent formylation at −40 °C of the highly reactive 2-bromophenyllithium intermediate using DMF, which is difficult to achieve by a conventional batch process due to the conversion of the highly reactive 2-bromophenyllithium intermediate into benzyne even at −78 °C. Late-stage cyclization to give the isoindolin-1-one ring system, through reductive amination of 18 followed by palladium-catalyzed carbonylation with carbon monoxide and intramolecular cyclization, efficiently afforded 1 for its further research and development.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...