Catalyst-free facile synthesis of polycyclic indole/pyrrole substituted-1,2,3-triazoles†
Abstract
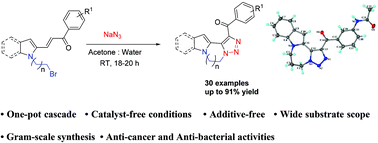
A general and catalyst-free access to the fused polycyclic N-heterocycles via an intramolecular azide–alkene cascade reaction under mild reaction conditions has been developed. The reaction is applicable to both indole and pyrrole substrates, and a variety of substituents are tolerated. The entire sequence can be carried out in a one-pot operation. This methodology provides a sustainable and efficient access to a variety of novel polycyclic indole/pyrrole substituted-1,2,3-triazoles.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...