Dendritic architectures by orthogonal thiol-maleimide “click” and furan-maleimide dynamic covalent chemistries†
Abstract
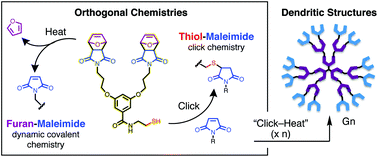
A set of dendrons and dendrimers is synthesized divergently using an orthogonal combination of kinetically-driven thiol-maleimide “click” chemistry and thermodynamically reversible furan-maleimide cycloaddition/retrocycloaddition reactions. Growth is controlled by taking advantage of the selective thiol–ene addition of thiols to the electron withdrawn alkene of maleimide in the presence of electron rich alkene of oxanorbornene. Subsequent activation of growing dendrons/dendrimers requires only heat to induce the dynamic covalent liberation of peripheral furan protecting groups. The methodology introduced provides a new route to multifunctional dendrimers that could, in principle, be synthesized by introducing different branched monomers at any stage of dendrimer growth, allowing dendrimer architectures and properties to be better tailored to their intended applications.


 Please wait while we load your content...
Please wait while we load your content...