Synthesis and electrochromic behavior of a multi-electron redox-active N-heteroheptacenequinone†
Abstract
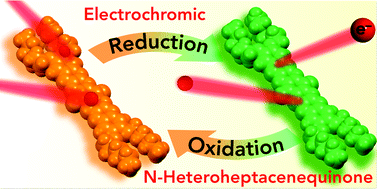
We report a novel N-heteroheptacenequinone derivative (C6OAHCQ) as a large π-conjugated framework. C6OAHCQ shows good electron-accepting behaviour owing to eight electron-deficient imino-N atoms and two carbonyl moieties and excellent solubility in common organic solvents. When a potential between 0 and −2.20 V is applied, C6OAHCQ is able to accept four electrons, which is more than fullerene C60 (three electrons) could accept in this voltage range. Moreover, a solution of C6OAHCQ and nBu4NPF6 in CH2Cl2 exhibits a clearly reversible brown-to-green colour change, suggesting that C6OAHCQ has potential as an electrochromic material.


 Please wait while we load your content...
Please wait while we load your content...