Direct thiocarbamation of imidazoheterocycles via dual C–H sulfurization†
Abstract
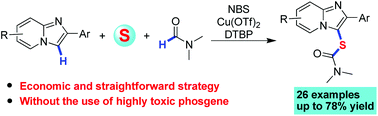
A copper-catalyzed DTBP oxidative dual C–H sulfurization has been developed for the direct thiocarbamation of imidazopyridines using a combination of elemental sulfur and formamides as carbamothioyl surrogates. NBS (bromo succinimide) was found to promote the thiocarbamation in good yields. This dual C–H sulfurization strategy enables access to a wide range of carbamothioyl imidazoheterocycles without the use of highly toxic phosgene.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...