Organocatalytic asymmetric spirocyclization reactions of cyclic 2,4-dienones with cyanoketones: synthesis of spiro-dihydropyrano cyclohexanones†
Abstract
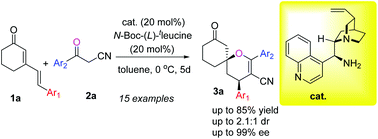
The first organocatalytic asymmetric synthesis of spiro-dihydropyrano cyclohexanones has been developed via the cascade reaction between cyanoketones and cyclic 2,4-dienones. A cinchona alkaloid-derived bifunctional primary amine catalyst in combination with N-Boc-tleucine was found to be the most effective for this spirocyclization reaction and provided the desired products in moderate to good yields with high enantioselectivities.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...