Abstract
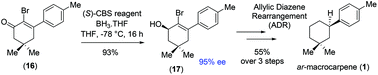
This report features the first catalytic asymmetric total synthesis of a sesquiterpene, (+)-ar-macrocarpene (1), in 7 steps with 42.1% overall yields from commercially available inexpensive 5,5-dimethylcyclohexane 1,3-dione. This strategy relies on a key [3,3]-sigmatropic rearrangement effecting reductive transposition through allylic diazene rearrangement (ADR) in a single step from intermediate allylic alcohol (+)-12 under the Mitsunobu reaction conditions with o-nitrobenzenesulfonyl hydrazide (o-NBSH). Enantioselective reduction of α-bromo vinylogous ester 16 under the Corey–Bakshi–Shibata reduction conditions forges the required stereocenter in the allylic alcohol (+)-12 in a highly enantioenriched manner (95% ee).
- This article is part of the themed collections: Synthetic methodology in OBC and Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...