Stereoselective construction of deoxy-cruciferane alkaloids by NHC-catalyzed intramolecular annulation of homoenolate with quinazolinone†
Abstract
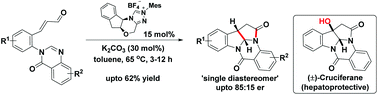
Chiral N-heterocyclic carbene (NHC)-catalyzed intramolecular [3 + 2] annulation of enals with an unactivated imine moiety of quinazolinone via formal homoenolate cycloaddition has been demonstrated. It is an excellent approach for stereoselective syntheses of deoxy-cruciferane alkaloids comprising a biologically important pyrroloindoline scaffold. Notably, this is the first report on the NHC-catalyzed asymmetric intramolecular homoenolate annulation with cyclic N-acyl amidine.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...