DFT studies on metal-catalyzed cycloisomerization of trans-1,5-enynes to cyclopropane sesquiterpenoids†
Abstract
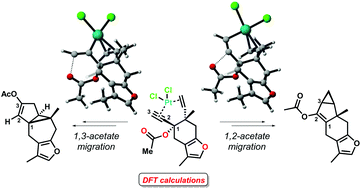
We have recently described the synthesis of strained carbocyclic sesquiterpenoid motifs through a highly regioselective cycloisomerization of common enyne acetates, in the presence of platinum(II) and gold(I) chlorides as catalysts. In this work, the mechanisms of these cyclization reactions have been studied by means of DFT methods. At the outset of the reactions, the propargyl substrates suffer 1,2- or 1,3-acetate rearrangements, which compete for the formation of a metal–carbene or a vinyl-metal species, respectively. These intermediates are the resting states of the cycles towards the formation of lindenane or myliol core structures. The DFT studies have revealed the energetics of the two migration processes, as well as the reasons for some of the key experimental observations, such as the syn/anti preference in the formation of the cyclopropane rings, the different reactivities of substrates containing furan or lactone moieties, and the different outcomes of the reactions when Pt(II) and Au(I) salts are used.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...