Highly regioselective α-formylation and α-acylation of BODIPY dyes via tandem cross-dehydrogenative coupling with in situ deprotection†
Abstract
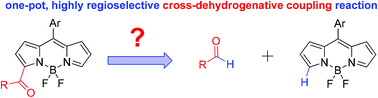
A metal-free C–H formylation and acylation of BODIPY dyes using a variety of dioxolane derivatives as aldehyde equivalents is reported, providing a postfunctionalization method for controllable synthesis of BODIPYs with carbonyl groups at 3,5-positions via a radical process. The photophysical properties of resultant dyes from this efficient one-pot, chemo- and site-selective transformation have been studied.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...