Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC)
Abstract
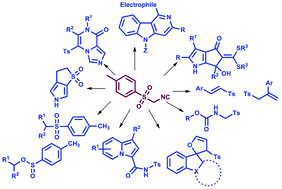
Since its introduction in 1972, TosMIC (p-toluenesulfonylmethyl isocyanide) has been recognised as an important building block in organic synthesis and a wide range of reaction strategies have been reported. This review article encompasses literature starting from 2011 and highlights the uniqueness of the TosMIC reagent in an assortment of synthetic methodologies. The advances in classical methodologies involving p-toluenesulfonylmethyl isocyanide which allow for the effortless synthesis of many simple/fused heterocycles via cycloaddition, regio- and stereo-selective, cascade/domino/tandem multicomponent, and metal catalyzed reactions and some natural products are presented in this review. This concise review provides a brief introduction about the reagent and its synthetic importance and flexibility in various reactions.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...