A rearrangement involving a solid-state melt reaction for the synthesis of multifunctional tetrasubstituted olefins†‡
Abstract
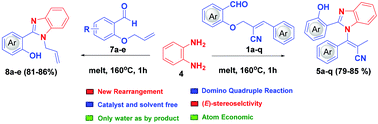
A catalyst- and solvent-free intramolecular rearrangement sequence leading towards benzimidazole-tethered tetrasubstituted olefins through a solid-state melt reaction (SSMR) involving imine formation, cyclization, N-allylation and isomerization has been realized for the first time. Interestingly, only water is the byproduct in this novel quadruple domino reaction. Furthermore, the reaction is highly stereoselective, atom economical and environmentally benign in nature.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...