Organic photoredox catalysis enabled cross-coupling of arenediazonium and sulfinate salts: synthesis of (un)symmetrical diaryl/alkyl aryl sulfones†
Abstract
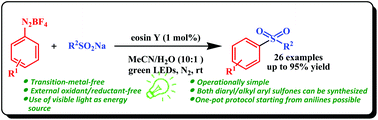
We disclose herein the first transition-metal- and external oxidant/reductant-free visible-light-mediated synthesis of (un)symmetrical diaryl/alkyl aryl sulfones from arenediazonium tetrafluoroborates and sodium sulfinates using eosin Y as an organic photoredox catalyst. The utilization of visible light as an inexpensive and ecosustainable energy source, operational simplicity, ambient temperature and clean reaction in aqueous acetonitrile are the salient features of the developed protocol. The desired sulfones were also synthesized via a one-pot, two-step process directly from anilines and sulfinate salts in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...