Superbase-promoted selective carbon–carbon bond cleavage driven by aromatization†
Abstract
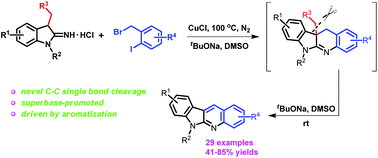
A novel selective carbon–carbon single bond cleavage has been disclosed through the copper-catalyzed reaction of 1-alkyl-3-alkylindolin-2-imine hydrochlorides with substituted 1-(bromomethyl)-2-iodobenzenes leading to fused N-heterocycles. Mechanistic studies showed that the intrinsic drive of aromatization and the action of the superbase derived from sodium tert-butoxide and dimethylsulfoxide were the key factors leading to the carbon–carbon single bond cleavage. Furthermore, the obtained N-heterocycles are indoloquinoline derivatives with wide biological activities.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...