Photorearrangement of dihetarylethenes as a tool for the benzannulation of heterocycles†
Abstract
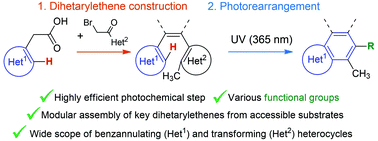
A general strategy for the preparative benzannulation of aromatic heterocycles via photocyclization of 1,2-dihetarylethenes was proposed for the first time. The strategy includes two steps, namely, modular assembly of dihetarylethenes from widely available 3-hetarylacetic acids and 2-bromo-1-hetarylethanones, and subsequent preparative photorearrangement (using a UV lamp at 365 nm as the light source). This approach is efficient for the annulation of a wide range of heterocycles and provides C-, N-, O- or S-substituents in the benzoheterocycles obtained. The photochemical step is a metal-, acid-, and oxidant-free reaction, which requires non-inert conditions, and can be easily monitored by NMR spectroscopy. Applicability of the proposed strategy was tested in the synthesis of a wide range of substituted carbazoles and benzo[b]thiophenes as well as on a gram-scale benzannulation of 3-indoleacetic acid. Our study disclosed how to overcome two notable obstacles to the successful photorearrangement of dihetarylethenes: undesired reactions associated with photogenerated singlet oxygen, and the instability of desired products. The first problem was successfully solved by the addition of DABCO, while development of an in situ alkylation protocol to trap unstable photoproducts allowed us to overcome the second issue.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...