Diversion of the Arbuzov reaction: alkylation of C–Cl instead of phosphonic ester formation on the fullerene cage†
Abstract
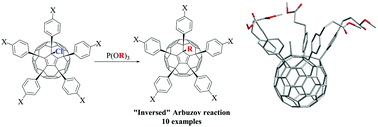
We report an “inversed” Arbuzov reaction of the fullerene derivatives C60Ar5Cl with trialkyl phosphites P(OR)3 producing alkylated fullerene derivatives C60Ar5R (R = Me, Et, iPr, nBu) with almost quantitative yields. This reaction provides a convenient synthetic route for the preparation of a large variety of functionalized fullerene derivatives with tailored properties, e.g. water-soluble compounds demonstrating promising antiviral activities against HCMV, HSV1, HIV and several influenza virus strains.


 Please wait while we load your content...
Please wait while we load your content...