The role of Brønsted base basicity in estimating carbon acidity at enzyme active sites: a caveat
Abstract
Many enzymes catalyze the abstraction of a proton from a carbon acid substrate to initiate a variety of reactions; however, the development of a complete quantitative description of enzyme-catalyzed heterolytic cleavage of a C–H bond remains a challenge to enzymologists. To determine the pKC–Ha value for such substrates bound at the active site, recent studies have estimated the equilibrium for formation of the deprotonated intermediate at the active site, however, accurate knowledge of the pKBH+a of the conjugate acid of the Brønsted base catalyst (BH+) is also required. Herein, it is shown that using the value of pKBH+a of the enzyme–substrate complex can underestimate the value of pKC–Ha by an amount between zero and pδ, where pδ is the change in basicity of BH+ upon going from the enzyme–substrate complex to the enzyme-intermediate complex.
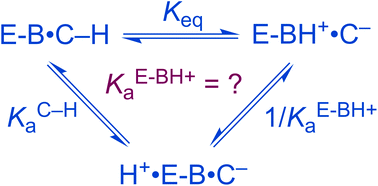
- This article is part of the themed collection: Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...