Acid–base controlled multiple conformation and aromaticity switches in tren-capped hexaphyrins†
Abstract
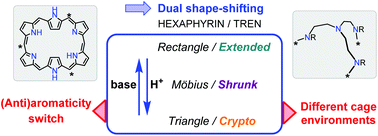
Upon protonation, a tren-capped hexaphyrin undergoes successive rectangular-to-Möbius and Möbius-to-triangular conformational isomerizations, with concomitant antiaromaticity-to-aromaticity reversal. This affords different cage environments leading ultimately to a “crypto-bowl-shape” hexaphyrin hosting a trifluoroacetate counterion.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...