BINOL-derived bifunctional sulfide catalysts for asymmetric synthesis of 3,3-disubstituted phthalides via bromolactonization†
Abstract
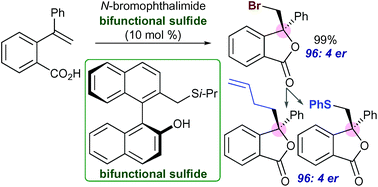
An efficient enantioselective synthesis of 3,3-disubstituted phthalides possessing a chiral quaternary carbon center was achieved via catalytic asymmetric bromolactonization that utilized BINOL-derived bifunctional sulfide catalysts. Transformations of the bromo group in optically active phthalide products were also performed to demonstrate the utility of this novel synthetic protocol.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...