Using Nature's polyenes as templates: studies of synthetic xanthomonadin analogues and realising their potential as antioxidants†
Abstract
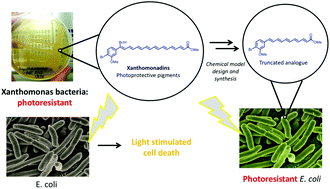
Two truncated analogues of the polyenyl photoprotective xanthomonadin pigments have been synthesised utilising an iterative Heck–Mizoroki (HM)/iododeboronation cross coupling approach and investigated as models of the natural product photoprotective agents in bacteria. Despite the instability of these types of compounds, both analogues proved to be sufficiently stable to allow isolation, spectroscopic analysis and biological studies of their photoprotective behaviour which showed that despite their shorter polyene chain length, they retained the ability to protect bacteria from photochemical damage; i.e. incorporation of one compound into E. coli provided photoprotective activity against singlet oxygen analogous to the natural photoprotective mechanisms employed by Xanthomonas bacteria, answering key questions about what minimal functionality is required to impart photoprotection, potentially leading to new classes of photoprotective and antioxidants compounds.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...