Playing supramolecular dominoes with light: building and breaking a photoreversible G-quadruplex made from guanosine, boric acid and an azobenzene†
Abstract
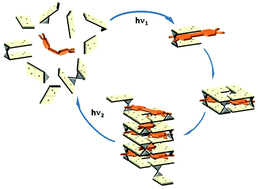
Addition of azobenzene-derivative 1 in its E configuration to an aqueous solution containing various guanosine borate esters induces a helical G-quartet based self-organization, stabilized by intercalation of the dye. The process is driven, in a domino fashion, by the initial host–guest interaction between the dye and a specific guanosine borate diester, whose structure can be thus assigned. This inclusion complex templates the formation of G-quartets. The quartets, in turn, pile up to form a supramolecular G-quadruplex structure, in which other G species present in solution are progressively included. The G-quadruplex can be reversibly broken and reformed by photoisomerization of the dye. This hierarchical and photosensitive self-assembly is unprecedented for simple guanosine derivatives.
- This article is part of the themed collection: Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...