Pyrrolidine ring puckering and prolyl amide bond configurations of 2-methyl-allo-hydroxyproline-based dipeptides†
Abstract
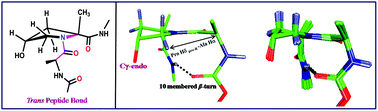
An expeditious method for the synthesis of homo and heterochiral dipeptides containing L-alanine and D/L 2-methyl allo-hydroxyl prolines was developed using direct aminolysis of bicyclic lactones derived from D/L alanine. The impact of C-2 methylation and its spatial orientation on the pyrrolidine ring puckering and prolyl amide bond configuration was ascertained by solution NMR studies. The present studies reveal that C-2 methylation causes the prolyl amide bond to exist exclusively in the trans geometry in both homo- and heterochiral dipeptides. However, the spatial orientation of the C-2 methyl group and its i + 2 position in appropriately capped model dipeptides may nucleate into a turn like structure.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...