Transition-metal-free C–C σ-bond activation of α-aryl ketones and subsequent Zn-catalyzed intramolecular cyclization: synthesis of tetrasubstituted furans†
Abstract
A highly atom-economical protocol for the synthesis of tetrasubstituted furans has been developed. This process is realized through the tandem reactions of Cs2CO3 promoted C–C σ-bond activation of α-aryl ketones followed by Zn-catalyzed intramolecular cyclization. This represents the first example for the preparation of tetrasubstituted furans through rearrangement of molecular skeletons and subsequent transformations. Mild reaction conditions and readily accessible starting materials make the protocol attractive in organic synthesis.
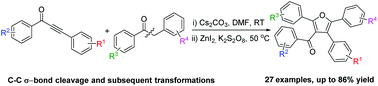
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...