Organocatalytic asymmetric Michael addition between 3-subsituted oxindoles and enals catalyzed by camphor sulfonyl hydrazine†
Abstract
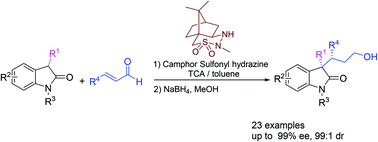
Organocatalytic asymmetric Michael addition between 3-substituted oxindoles and enals catalyzed by chiral camphor sulfonyl hydrazines (CaSHs) has been developed. A wide range of 3-substituted oxindoles and enals were successfully used, giving the corresponding 3,3-disubstituted oxindoles containing vicinal stereogenic carbon centers in good yields with good to excellent enantioselectivities and moderate to good diastereoselectivities (up to 89% yield, 99% ee and 99 : 1 dr).
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...