Crystal structure of LepI, a multifunctional SAM-dependent enzyme which catalyzes pericyclic reactions in leporin biosynthesis†
Abstract
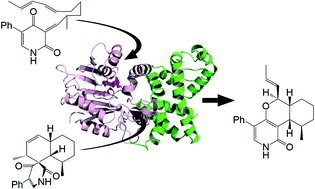
LepI is a novel multifunctional enzyme that catalyzes stereoselective dehydration, Diels–Alder reaction, and retro-Claisen rearrangement. Here we report the crystal structure of LepI in complex with its co-factor S-adenosyl methionine (SAM). LepI forms a tetramer via the N-terminal helical domain and binds to a SAM molecule in the C-terminal catalytic domain. The binding modes of various LepI substrates are investigated by docking simulations, which suggest that the substrates are bound via both hydrophobic and hydrophilic forces, as well as cation–π interactions with the positively charged SAM. The reaction starts with a dehydration step in which H133 possibly deprotonates the pyridone hydroxyl group of the substrate, while D296 might protonate an alkyl-chain hydroxyl group. Subsequent pericyclization may be facilitated by the correct fold of the substrate's alkyl chain and a thermodynamic driving force towards σ-bonds at the expense of π-bonds. These results provide structural insights into LepI catalysis and are important in understanding the mechanism of enzymatic pericyclization.


 Please wait while we load your content...
Please wait while we load your content...