Conversion of bimetallic PtNi3 nanopolyhedra to ternary PtNiSn nanoframes by galvanic replacement reaction†
Abstract
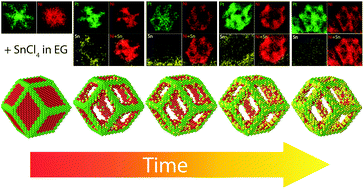
Hollow multimetallic PtNiSn nanoparticles (NPs) were formed from solid Ni-core/Pt-frame NPs by the galvanic replacement reaction (GRR) of Ni by Sn. The GRR was performed by adding SnCl4·5H2O dissolved in ethylene glycol into the PtNi3 NPs containing suspension. The reaction yielded nanoframes with a hollow interior, having Pt-rich edges covered with a thin, incomplete Sn layer. They were investigated using transmission electron microscopy (TEM), energy dispersion X-ray spectroscopy (EDS) and X-ray diffraction (XRD). EDS analysis showed that the GRR rate could be modified by changing the solvent and the concentration of tin ions. Indeed, compared to water, ethylene glycol was found to facilitate the reduction of tin chloride and to affect nickel dissolution. TEM analysis revealed that the galvanic replacement of nickel and tin involves two different mechanisms. The first one consists of nickel oxidation followed by reduction of tin ions. In the second mechanism, oxidation of nickel and reduction of tin ions occur simultaneously.


 Please wait while we load your content...
Please wait while we load your content...