Porous PdRh nanobowls: facile synthesis and activity for alkaline ethanol oxidation†
Abstract
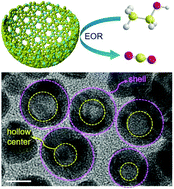
Optimizing structure and composition with respect to electrocatalytic performance is critical to achieve outstanding Pd-based electrocatalysts. Herein, we have successfully developed a novel electrocatalyst of hollow and porous PdRh nanobowls (PdRh NBs) for the ethanol oxidation reaction (EOR) by using urea as a guiding surfactant. Under alkaline hydrothermal conditions, urea molecules can release bubbles (NH3 and CO2) that in turn guide the formation of PdRh nanobowls. The porous bowl-like structures of PdRh NBs expose abundant surface sites, which allows for increased collision frequency via confining reactants within open spaces. In regards to composition, the reason for introducing Rh is that not only is the redox potential of Rh approximate with that of Pd (beneficial to the formation of high PdRh alloy phase), but also it can effectively facilitate the breakage of C–C bond on the electrode surface (enhancing the total oxidation of ethanol to CO2). Benefiting from the compositional and structural advantages, the newly developed PdRh NBs exhibit significantly improved electrocatalytic activity for the EOR compared with those of the pure Pd NBs, PdRh nanoparticles (PdRh NPs) and commercial Pd black. These attributes might make them good anodic candidates for application in direct ethanol fuel cells.


 Please wait while we load your content...
Please wait while we load your content...