Pd/C-catalyzed synthesis of oxamates by oxidative cross double carbonylation of alcohols and tertiary amines through C–N bond cleavage†
Abstract
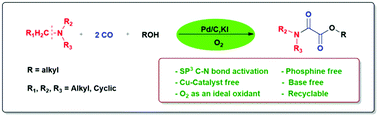
An efficient synthesis of oxamates by Pd/C-catalyzed oxidative cross double carbonylation of alcohols and unactivated tertiary amines has been demonstrated. The in situ oxidative C–N bond cleavage of tertiary amines was achieved using molecular oxygen as an environmentally benign oxidant providing a user-friendly approach to the synthesis of oxamates. The developed protocol showed excellent activity towards the cyclic as well as aliphatic tertiary amines and long-chain alcohols. This developed protocol system is advantageous, as it is phosphine ligand free, base free, and copper-free and the Pd/C catalyst is easily recyclable. The Pd/C catalyst was recycled up to five consecutive cycles.


 Please wait while we load your content...
Please wait while we load your content...