Copper-catalyzed selective difunctionalization of N-heteroarenes through a halogen atom transfer radical process†
Abstract
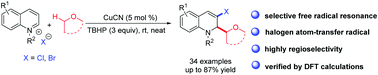
A highly regioselective Cu-catalyzed difunctionalization of different N-heteroarene salts such as quinolinium and benzothiazolim salts was developed with ether and X− (X = Br, Cl) as the halogen source under mild conditions. This transformation involved the combination of oxidative coupling, selective free radical resonance and a copper-catalyzed halogen atom-transfer radical process. The regiochemistry was further verified by DFT calculations. This method not only provided an efficient way to prepare various substituted azaarenes but also achieved the selective construction of C(sp2)–X (X = Br, Cl) bonds from a halogen anion and nucleophilic carbon atom via a free-radical process.


 Please wait while we load your content...
Please wait while we load your content...