3-Trinitromethyl-4-nitro-5-nitramine-1H-pyrazole: a high energy density oxidizer†
Abstract
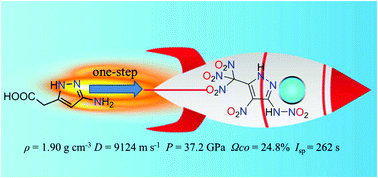
A novel oxygen-rich energetic compound, 3-trinitromethyl-4-nitro-5-nitramine-1H-pyrazole (5), was obtained by one-step nitration of 2-(3-amino-1H-pyrazol-5-yl)acetic acid (4). Compound 5 was characterized by IR and NMR spectroscopy, elemental analysis and differential scanning calorimetry (DSC). Additionally, the structure of compound 5 was further confirmed by X-ray crystallography. Compound 5 has a high density (1.90 g cm−3), positive heat of formation (0.99 kJ g−1) and positive oxygen balance (24.8%). The calculated detonation velocity (D = 9124 m s−1) and pressure (P = 37.2 GPa) are superior to those of RDX (D = 8795 m s−1, P = 34.9 GPa). The calculated specific impulse of compound 5 (262 s) is higher than that of AP (157 s) and ADN (202 s). The combination of these attractive properties makes it a promising high energy density oxidizer.


 Please wait while we load your content...
Please wait while we load your content...