Studies of air-exposure effects and remediation measures on lithium bis(oxalato)borate
Abstract
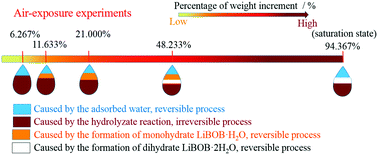
The impurity of water, which should be controlled strictly during industrial production and storage processes, has a fatal influence on the performance of lithium ion batteries. Here, lithium bis(oxalato)borate is taken as an example to investigate the changes in properties of chelate-borate complexes when stored in a humid environment, using techniques of attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR), thermogravimetric (TG) analysis and density functional theory (DFT) calculations. Besides, the effect of heating on remediation is studied with acidity test and electrochemical performance measurement. Results show that high relative humidity can accelerate the rate of increase in weight, though the same constant weight will be achieved in the end. The increase in weight is caused by a comprehensive action including the occurrence of hydrolytic reactions, and the formation of adsorbed water and crystal water. Further studies indicate that hydrolytic decomposition is spontaneous at room temperature, but the backward reaction cannot proceed with a heating method, namely, the remediation will be limited due to the formation of harmful hydrolysis products, though both the adsorbed water and the crystal water can be removed thoroughly. Moreover, we believe that the existence of hydrolysis products will lead to a sharp impedance increase of the so-called solid electrolyte interphase film, which will give rise to the deterioration of electrochemical performance sequentially.


 Please wait while we load your content...
Please wait while we load your content...