Selective sensing of a Cu(ii) ion by organotin anchored keto-enamine ligands†
Abstract
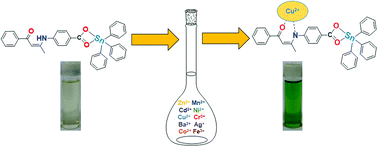
Three new organotin carboxylates [{(n-Bu)2Sn(HL)}2O]2 (1), [(t-Bu)2Sn(HL)2] (2) and [Ph3Sn(HL)] (3) where n-Bu = n-butyl, t-Bu = t-butyl, Ph = phenyl and HL = deprotonated form of (Z)-1-phenyl-3-(4-carboxyphenylamino)-1-phenylbut-2-en-1-one (H2L) have been prepared. Structural analysis shows that complex 1 contains a tetranuclear organodistannoxane unit with two crystallographically independent tin(IV) centres. Complexes 2 and 3 are monomers containing a six- and five-coordinated tin(IV) centre, respectively. All complexes are further characterized by multi-nuclear NMR spectroscopy, elemental analysis, and FT-IR spectroscopy. All complexes selectively sense Cu(II) ions among various metal ions. Complex 3 exhibits high binding constant (0.4 × 105 M−1) and a better limit of detection value (1.47 × 10−6 M) than the other complexes.


 Please wait while we load your content...
Please wait while we load your content...