Environmentally friendly synthesis of unsymmetrical dialkyl disulfides by reacting organic halides with thiourea and sodium thiosulfate in an aqueous medium†
Abstract
Owing to the current environmental awareness, the development of environmentally friendly reactions in aqueous media is the subject of an increasing number of studies. Hence, we report a novel three-component reaction of alkyl halides, thiourea and Bunte salts using non-toxic H2O as a benign reaction medium. After heating for 7 h, various unsymmetrical disulfides in high purity could be prepared in up to 92% yield. The substrates are readily available and the protocol does not require either extra oxidant or reductant. In addition, no transition metal catalyst was required, making this process environmentally and economically friendly.
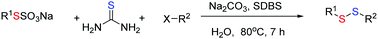


 Please wait while we load your content...
Please wait while we load your content...