Ligand's electronegativity controls the sense of enantioselectivity in BIFOP-X palladium-catalyzed allylic alkylations†
Abstract
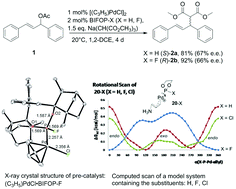
Palladium-catalyzed allylic alkylations of sodium dimethyl malonate with 1,3-diphenylallyl acetate, employing BIFOP-H (biphenylbisfencholphosphite) and analogue (i.e. BIFOP-X, X = D, Cl, CN, N3) ligands, all yield (S)-enantiomeric products, while alkylations to cyclohexenyl acetate yield the (R)-enantiomeric C–C coupling product (up to 91% yield, 70% ee). The fluoro derivative BIFOP-F however, “switches” the sense of enantioselectivity, yielding the (R)-enantiomer for 1,3-diphenylallyl acetate and the (S)-enantiomer for the cyclohexenyl acetate (up to 92% yield, 67% ee). Computational analyses of transition structures (M06-2X-D3/def2-TZVP//B3LYP-D3(BJ)/def2-SVP) for these Pd-catalyzed allylic alkylations reproduce the experimental preference of BIFOP-H (and analogue BIFOP-X ligands) for (R)- or (S)-enantiomeric products of 1,3-diphenylallyl or cyclohexenyl acetate, respectively. The “F-switch” of the sense of enantioselectivity from BIFOP-H to BIFOP-F is also apparent computationally and is found (NBO-analyses) to originate from lp(Pd) → σ*(P–O) or lp(Pd) → σ*(P–F) hyperconjugations. The higher electronegativity of F vs. H in BIFOP-X hence controls the sense of enantioselectivity of this Pd-catalyzed allylic alkylation.


 Please wait while we load your content...
Please wait while we load your content...