Palladium-catalysed regioselective N-arylation of anthranilamides: a tandem route for dibenzodiazepinone synthesis†
Abstract
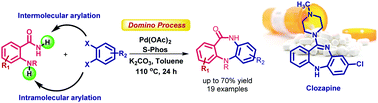
A palladium-catalyzed domino approach to the synthesis of 10,11-dihydro-5H-dibenzo[b,e][1,4]diazepinones from 2-aminobenzamides and 1,2-dihaloarenes has been developed. Our strategy integrating double N-arylations (inter- and intra-molecular) of 2-aminobenzamides with 1,2-dihaloarenes under palladium-catalyzed conditions is clearly distinct from the current literature available for the synthesis of dibenzodiazepinones. Unlike a previous report described for regioselective N-arylation of 2-aminobenzamide at the amine group, our mechanistic studies support the regioselective N-arylation of 2-aminobenzamide occurring first primarily at the amide group. The translational application of our protocol may be demonstrated in the synthesis of a marketed drug, clozapine.


 Please wait while we load your content...
Please wait while we load your content...