Two-step etherification of phenolic-oil with methanol under catalysis of alumina-supported metal salts†
Abstract
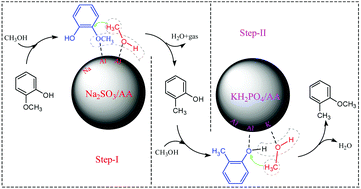
A novel two-step process for the etherification of phenolic-oil was put forward to avoid the hindering effect of alkoxyphenols on the etherification of alkylphenols. In the first step, the phenolic-oil with both alkylphenols and alkoxyphenols was converted to the primary product rich in alkylphenols. In the second step, the primary product oil was further converted to arylether-enriched oil by the etherification of alkylphenols with methanol. For the first step, Na2SO3/Al2O3 was found to be the best catalyst among 7 tested alumina-supported metal salts. The reaction conditions were optimized as 500 °C with a GHSV of 1944 h−1, under which alkoxyphenols almost completely disappeared and the content of alkylphenols reached up to 70.07%. For the second step with the catalyst KH2PO4/Al2O3, a high content of arylethers (62.87%) was obtained in the product oil under the optimized conditions of 450 °C with a GHSV of 1819 h−1. The characterization of the catalysts showed that Na5AlO4 was generated in the catalyst Na2SO3/Al2O3 after calcination at 700 °C for 8 h, and Na5AlO4 was verified as the essential substance in promoting the selective conversion of alkoxyphenols to alkylphenols. Acid sites with stronger acidity favored the conversion of alkoxyphenols to alkylphenols, while the acid sites with weaker acidity were more beneficial for the etherification of alkylphenols. Additionally, the catalysts with transition metals like Fe and Co further promoted the reaction of hydrodeoxygenation.


 Please wait while we load your content...
Please wait while we load your content...