Versatility and trends in the interaction between Pd(ii) and peptide hydroxamic acids†
Abstract
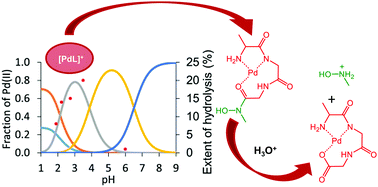
Primary and secondary di- and tripeptide hydroxamic acids, Ala-Ala-NHOH, Ala-Ala-N(Me)OH, Ala-Gly-Gly-NHOH and Ala-Gly-Gly-N(Me)OH were synthesized and their interaction with Pd(II) (as a Pt(II) model but with faster ligand exchange reactions) was studied in aqueous solution in the presence of the Cl− competitor ion by pH-potentiometric and 1H NMR methods. To the best of our knowledge, this is the first detailed solution study on Pd(II)–peptide hydroxamate systems revealing that, except for Ala-Gly-Gly-NHOH, the other three ligands act not only as coordination compounds, but also the hydrolysis of the coordinated ligands and formation of the protonated hydroxylamine and Pd(II) complexes of the corresponding peptides under acidic conditions occurred. The hydrolysis was rather slow with Ala-Gly-Gly-N(Me)OH (more than one week), and just a bit faster with Ala-Ala-NHOH, so speciation studies could also be performed successfully on the systems containing one of the latter two ligands. This was, however, hindered for the Pd(II)–Ala-Ala-N(Me)OH system, where, in addition to the quite fast hydrolysis of the ligand, the reduction of Pd(II) to elementary metal by the N(Me)-hydroxylamine formed was also observed. Speciation studies with Ala-Gly-Gly-NHOH revealed the predominance of a very stable 4N-donor complex, (NH2, 2Namide, Nhydr.) over a wide pH range. This ligand is also capable of binding the metal ion excess with the hydroxymate (O,O) set in dinuclear species. The formation of this latter type of complex is hindered with the secondary analogue, Ala-Gly-Gly-N(Me)OH, where, in addition to the 3N donor atoms, the hydroxamate-O is also involved in the coordination of the most stable complex. However, the formation of mixed hydroxo species at high pH and a bis-complex in a rather slow process with (NH2, Namide)2 bonding mode in the presence of ligand excess was proven. Although the 3N coordination (NH2, Namide, Nhydx) results in a highly stable complex with the dipeptide derivative, Ala-Ala-NHOH, the fourth coordination site remains free for accepting an NH2 moiety from the excess ligand, or a hydroxide ion at high pH. Likewise, the hydroxymate (O,O) set remains free to bind the metal ion excess in a trinuclear species. The results of this study may also contribute to the design and synthesis of novel Pt(II) complexes with anticancer potential.


 Please wait while we load your content...
Please wait while we load your content...