Cu(ii) and Zn(ii) complexes with a poly-functional ligand derived from o-vanillin and thiophene. Crystal structure, physicochemical properties, theoretical studies and cytotoxicity assays against human breast cancer cells†
Abstract
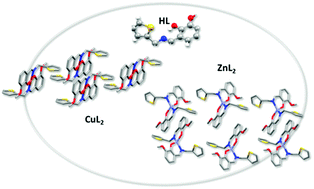
The interaction of a poly-functional ligand derived from o-vanillin and 2-thiophenemethylamine (oVATPNH2) with transition metal ions Cu(II) and Zn(II) leads to the formation of stable coordination compounds, namely [Cu(oVATPNH2)2] and [Zn(oVATPNH2)2]. Their crystal structures have been determined by X-ray diffraction methods. Two molecules of the deprotonated ligand acting in a bidentate fashion build a nearly square planar environment around Cu(II) and a distorted tetrahedral coordination arrangement for Zn(II). The complexes were characterized by spectroscopic techniques, including solid state FTIR, Raman, EPR and diffuse reflectance and solution UV-vis and EPR. Their thermal behavior has been analyzed by means of TGA and DTA. DFT theoretical studies, using computational methods based on DFT, were employed to assist the interpretation and assignment of spectroscopic data. Cytotoxicity assays against two human breast cancer cell lines, namely MCF-7 and MDA-MB-231, revealed an enhancement of the effectiveness of the complexes as compared with both the ligand and the free metal ions. The results for the copper compound are promising, as its cytotoxic effect was stronger than the reference metallodrug cisplatin in both cancer cell lines tested.


 Please wait while we load your content...
Please wait while we load your content...