A viscochromic, mechanochromic, and unsymmetrical azine for selective detection of Al3+ and Cu2+ ions and its mitotracking studies†
Abstract
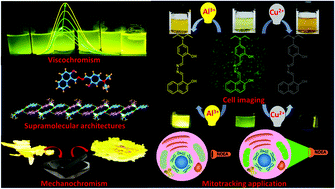
A hydrazone obtained by the reaction of 2-hydroxynaphthaldehyde with hydrazine hydrate was allowed to react further with 4-diethylamino-2-hdroxybenzaldehyde to provide an unsymmetrical azine derivative (NDEA). The azine has been characterized using spectroscopic techniques and single crystal X-ray crystallography. It selectively detects Al3+ and Cu2+ ions in aqueous methanol (DMSO : H2O : MeOH = 0.1 : 1.9 : 8.0, v/v, HEPES buffer, pH 7.4) and exhibits naked eye visible color changes from greenish yellow to bright yellow and brown on the addition of Al3+ and Cu2+ ions, respectively, with concomitant changes in their absorption spectra. The corresponding solutions containing Al3+ and Cu2+ ions also display changes in their emission spectra via “TURN ON” and “TURN OFF” pathways with a detection limit of 1.65 × 10−7 M and 1.52 × 10−7 M, respectively. NDEA works as a reversible probe towards Al3+ ions and Cu2+ ions by the addition of F− ions and EDTA2− ions, respectively. The complexes of NDEA, separately with Al3+ and Cu2+ ions, have been optimized by DFT (density functional theory). It also exhibits viscochromic and mechanochromic properties. Most importantly, NDEA significantly detects these ions in rat C6 glioma cell lines and displays good cell permeability. It also co-localizes with the commercially available Mitotracker Red and exhibits a unique application as a live cell mitochondrial tracker. Practically, NDEA detects Al3+ and Cu2+ ions in real water samples and blood serum with a high accuracy.


 Please wait while we load your content...
Please wait while we load your content...