Binding aspects of dietary flavone, luteolin, with polymorphic forms of natural DNA: a spectroscopic and molecular docking approach
Abstract
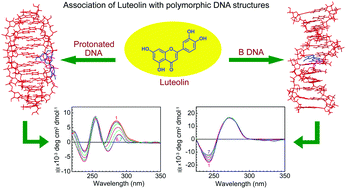
Physicochemical studies on the interactions of small molecules with different polymorphs of DNA are relevant for elucidation at the molecular level of the processes occurring in vivo. In our work, various spectroscopic techniques combined with molecular modeling computations have been used to explore the interactions of luteolin (LTN), a pharmacologically important bioactive flavone, with two polymorphic forms of natural DNA. These include the low pH induced left-handed protonated and right-handed B forms of DNA in aqueous buffer medium. The association was characterized by hypochromic and red shifted absorption maxima, enhanced fluorescence intensity and strong perturbation in the circular dichroism (CD) spectra. Binding parameters along with significant thermal stabilization indicate a greater extent of association of LTN with the protonated form in comparison to the native B form structure of DNA. Viscometric and molecular docking studies together infer that the binding mode of LTN to the protonated DNA is external stacking while with B DNA it is intercalation.


 Please wait while we load your content...
Please wait while we load your content...