Selective chemotherapy and imaging of colorectal and breast cancer cells by a modified MUC-1 aptamer conjugated to a poly(ethylene glycol)-dimethacrylate coated Fe3O4–AuNCs nanocomposite†
Abstract
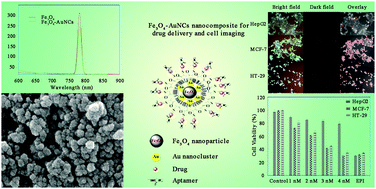
The combination of diagnosis and targeted therapy within a single nanoplatform is one of the remarkable advances in molecular medicine. Herein, we synthesized a magnetite–gold nanocluster–aptamer(epirubicin) (Fe3O4–AuNC–Apt(EPI)) nanocomposite with an average particle size of 35 nm for specific detection, fluorescent imaging, and therapy of colorectal (HT-29) and breast (MCF-7) cancer cells. AuNCs were synthesized in situ in (γ-mercaptopropyl) trimethoxysilane (MPS) solution to produce a highly fluorescent nanocomposite. In this study, short-length poly(ethylene glycol)-dimethacrylate (PEGDMA) was used for efficient immobilization of further aptamer on the nanocomposite along with the reduction of the growth rate of the silica network and thickness of the coating, subsequently. A thiol-modified MUC-1 aptamer (Apt) with an increased CG base pair region was conjugated to the surface of the nanocomposite which provides the situation for a high amount of epirubicin (EPI) loading as a chemotherapeutic drug specifically targeted toward the desired cancer cells. Also, the calculated binding free energies (ΔGb) showed that the binding affinity of EPI to the active sequence modified Apt was higher than the traditional one. According to the MTS results, this nanocomposite is able to kill the MUC-1 positive cancer cells (HT-29 and MCF-7) more efficiently than MUC-1 negative cells (HepG2) which shows its selective behavior when compared to the free EPI. Surprisingly, the toxicity threshold of EPI increased by 1.5 times using the nanocomposite. Taking advantage of imaging and chemotherapy, this nanocomposite is able to detect HT-29 and MCF-7 specifically as MUC-1 expressing cancer cells and release EPI inside of them.


 Please wait while we load your content...
Please wait while we load your content...