Piperidine carbamate peptidomimetic inhibitors of the serine proteases HGFA, matriptase and hepsin†
Abstract
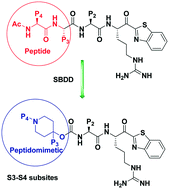
Matriptase and hepsin are type II transmembrane serine proteases (TTSPs). Along with related S1 trypsin like serine protease HGFA (hepatocyte growth factor activator), their unregulated proteolytic activity has been associated with cancer including tumor progression and metastasis. These three proteases have two substrates in common, hepatocyte growth factor (HGF) and macrophage stimulating protein (MSP), the ligands for MET and recepteur d'origine nantais (RON) receptor tyrosine kinases. Mechanism-based tetrapeptide and benzamidine inhibitors of these proteases have been shown to block HGF/MET and MSP/RON cancer cell signaling. Herein, we have rationally designed a new class of peptidomimetic hybrid small molecule piperidine carbamate dipeptide inhibitors comparable in potency to much larger tetrapeptides. We have identified multiple compounds which have potent activity against matriptase and hepsin and with excellent selectivity over the off-target serine proteases factor Xa and thrombin.


 Please wait while we load your content...
Please wait while we load your content...