Synthesis and antitumor activity of aza-brazilan derivatives containing imidazolium salt pharmacophores†
Abstract
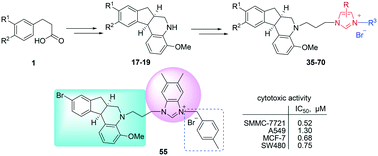
The synthesis of a series of novel aza-brazilan derivatives containing imidazolium salt pharmacophores is presented. The biological activity of such imidazolium salts was further evaluated in vitro against a panel of human tumor cell lines. The results suggest that the electron-withdrawing group on the aza-brazilan moiety, substituted 5,6-dimethyl-benzimidazole ring and substitution of the imidazolyl-3-position with a 4-methylbenzyl group were essential for modulating the cytotoxic activity. Compounds 55 and 39, bearing a 4-methylbenzyl substituent at position-3 of 5,6-dimethyl-benzimidazole, were found to be the most potent compounds with IC50 values of 0.52–1.30 μM and 0.56–1.51 μM against four human tumor cell lines investigated. Particularly, compound 57 exhibited inhibitory activity against the MCF-7 cell line with an IC50 value of 0.35 μM and was 56-fold more sensitive than DDP. Moreover, compound 55 inhibited cell proliferation through inducing G0/G1 cell cycle arrest and apoptosis in SMMC-7721 cells.


 Please wait while we load your content...
Please wait while we load your content...