Design, synthesis and biological evaluation of novel 2-phenyl pyrimidine derivatives as potent Bruton's tyrosine kinase (BTK) inhibitors†
Abstract
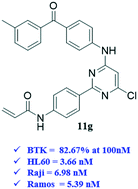
BTK is an effective target for the treatment of B-cell malignant tumors and autoimmune diseases. In this work, a series of 2-phenyl pyrimidine derivatives were prepared and their preliminary in vitro activities on B-cell leukemia cells as well as the BTK enzyme were determined. The results showed that compound 11g displayed the best inhibitory activity on BTK with an inhibition rate of 82.76% at 100 nM and excellent anti-proliferation activity on three B-cell leukemia lines (IC50 = 3.66 μM, 6.98 μM, and 5.39 μM against HL60, Raji and Ramos, respectively). Besides, the flow cytometry analysis results indicated that 11g inhibited the proliferation of the Raji cells in a dose- and time-dependent manner, and blocked the Ramos cells at the G0/G1 phase, which is in accordance with the positive control ibrutinib. The mechanism investigation demonstrated that 11g could inhibit the phosphorylation of BTK and its downstream substrate phospholipase γ2 (PLCγ2). All these results showed that 11g was a promising lead compound that merited further optimization as a novel class of BTK inhibitor for the treatment of B-cell lymphoblastic leukemia.


 Please wait while we load your content...
Please wait while we load your content...