Methoxy substituted 2-benzylidene-1-indanone derivatives as A1 and/or A2A AR antagonists for the potential treatment of neurological conditions†
Abstract
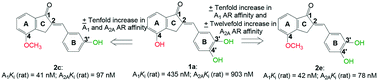
A prior study reported on hydroxy substituted 2-benzylidene-1-indanone derivatives as A1 and/or A2A antagonists for the potential treatment of neurological conditions. A lead compound (1a) was identified with both A1 and A2A affinity in the micromolar range. The current study explored the structurally related methoxy substituted 2-benzylidene-1-indanone derivatives with various substitutions on ring A and B of the benzylidene indanone scaffold in order to enhance A1 and A2A affinity. This led to compounds with both A1 and A2A affinity in the nanomolar range, namely 2c (A1Ki (rat) = 41 nM; A2AKi (rat) = 97 nM) with C4-OCH3 substitution on ring A together with meta (3′) hydroxy substitution on ring B and 2e (A1Ki (rat) = 42 nM; A2AKi (rat) = 78 nM) with C4-OCH3 substitution on ring A together with meta (3′) and para (4′) dihydroxy substitution on ring B. Additionally, 2c is an A1 antagonist. Consequently, the methoxy substituted 2-benzylidene-1-indanone scaffold is highly promising for the design of novel A1 and A2A antagonists.


 Please wait while we load your content...
Please wait while we load your content...